When considering the balanced equation of aluminum reacting with sulfuric acid, 2 Al + 3 H₂SO₄ → Al₂(SO₄)₃ + 3 H₂, we need to count the atoms of each element on both sides of the equation. The reactants have 2 aluminum (Al) atoms, 6 hydrogen (H) atoms, and 3 sulfuric acid molecules which total 3 sulfur (S) atoms and 12 oxygen (O
Al+H2SO4=Al2(SO4)3+H2. balance the chemical equation @mydocumentary838. – YouTube
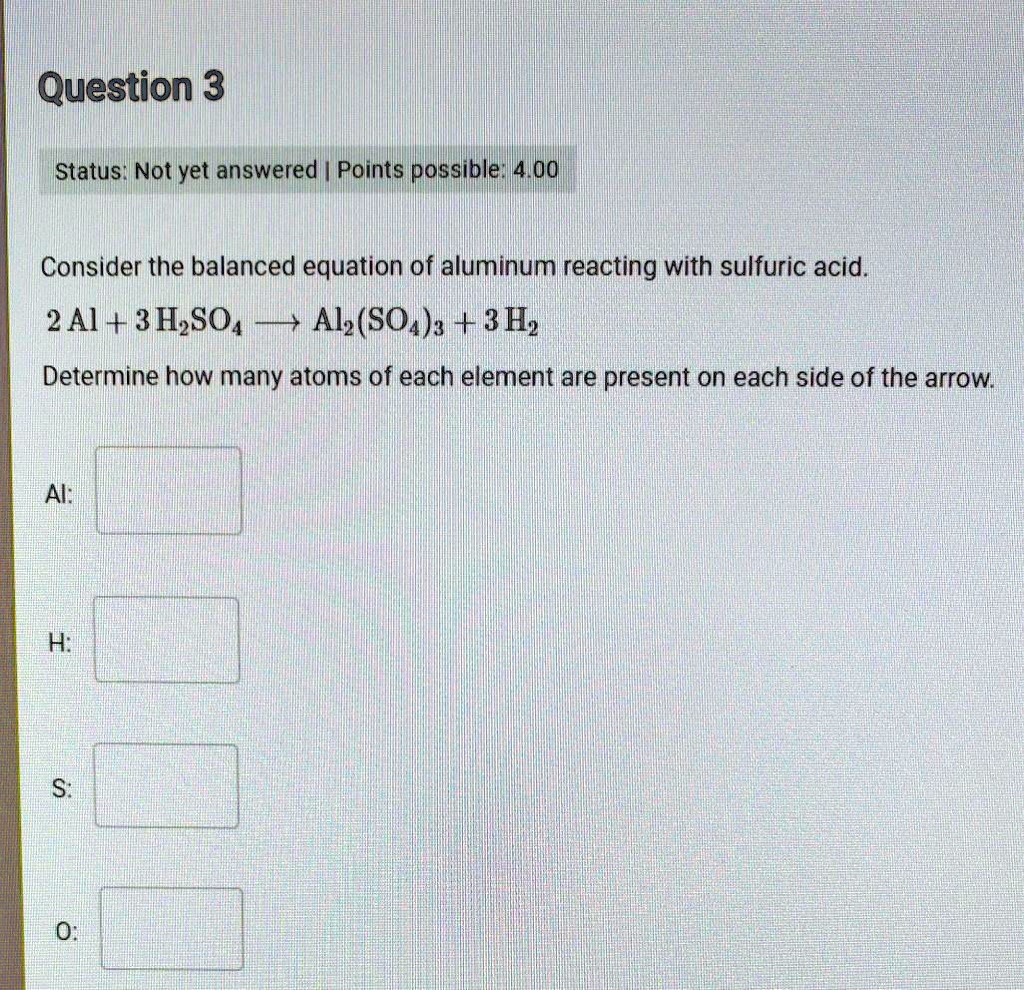
Chemistry Chemistry questions and answers Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 + Al2 (SO4)3 + 3H2 Determine how many atoms of each element are present on each side of the arrow. Al: 4 H: 12 (X) S: 6 X 0: 24 This problem has been solved!
Source Image: melscience.com
Download Image
1 Answer Meave60 Apr 20, 2018 Balanced Equation: 2Al (s) +3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g) Explanation: Al (s) + H2SO4(aq) → Al2(SO4)3(aq) + H2(g) Start with the polyatomic sulfate ion, SO4. There is one sulfate ion on the left-hand side and there are three on the right-hand side. Place a coefficient of 3 in front of H2SO4.

Source Image: studypool.com
Download Image
How to Write the Net Ionic Equation for Al + H2SO4 = Al2(SO4)3 + H2 – YouTube Related questions Question Explain this Transcribed Image Text: Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 → Al2 (SO4)3 + 3 H2 Determine how many atoms of each element are present on each side of the arrow. Al: H: S: O: Expert Solution Trending now This is a popular solution! Step by step

Source Image: m.youtube.com
Download Image
Consider The Balanced Equation Of Aluminum Reacting With Sulfuric Acid
Related questions Question Explain this Transcribed Image Text: Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 → Al2 (SO4)3 + 3 H2 Determine how many atoms of each element are present on each side of the arrow. Al: H: S: O: Expert Solution Trending now This is a popular solution! Step by step Science Chemistry Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 Al (SO4)3 + 3 H2 Determine how many atoms of each element are present on each side of the arrow.
How to Balance Al2O3 + H2SO4 = Al2(SO4)3 + H2O (Aluminum oxide + Sulfuric acid) – YouTube
Aluminium + Sulfuric Acid = Aluminum Sulfate + Dihydrogen Al + H2SO4 = Al2 (SO4)3 + H2 is a Single Displacement (Substitution) reaction where two moles of solid Aluminium [Al] and three moles of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Aluminum Sulfate [Al 2 (SO 4) 3] and three moles of Dihydrogen [H 2] gas SOLVED: Consider the balanced equation of aluminum reacting with sulfuric acid: 2Al + 3 H2SO4 → Al2(SO4)3 + 3 H2. Determine how many atoms of each element are present on each side
Source Image: numerade.com
Download Image
Solved Below is the balanced equation showing the reaction | Chegg.com Aluminium + Sulfuric Acid = Aluminum Sulfate + Dihydrogen Al + H2SO4 = Al2 (SO4)3 + H2 is a Single Displacement (Substitution) reaction where two moles of solid Aluminium [Al] and three moles of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Aluminum Sulfate [Al 2 (SO 4) 3] and three moles of Dihydrogen [H 2] gas
Source Image: chegg.com
Download Image
Al+H2SO4=Al2(SO4)3+H2. balance the chemical equation @mydocumentary838. – YouTube When considering the balanced equation of aluminum reacting with sulfuric acid, 2 Al + 3 H₂SO₄ → Al₂(SO₄)₃ + 3 H₂, we need to count the atoms of each element on both sides of the equation. The reactants have 2 aluminum (Al) atoms, 6 hydrogen (H) atoms, and 3 sulfuric acid molecules which total 3 sulfur (S) atoms and 12 oxygen (O

Source Image: youtube.com
Download Image
How to Write the Net Ionic Equation for Al + H2SO4 = Al2(SO4)3 + H2 – YouTube 1 Answer Meave60 Apr 20, 2018 Balanced Equation: 2Al (s) +3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g) Explanation: Al (s) + H2SO4(aq) → Al2(SO4)3(aq) + H2(g) Start with the polyatomic sulfate ion, SO4. There is one sulfate ion on the left-hand side and there are three on the right-hand side. Place a coefficient of 3 in front of H2SO4.

Source Image: m.youtube.com
Download Image
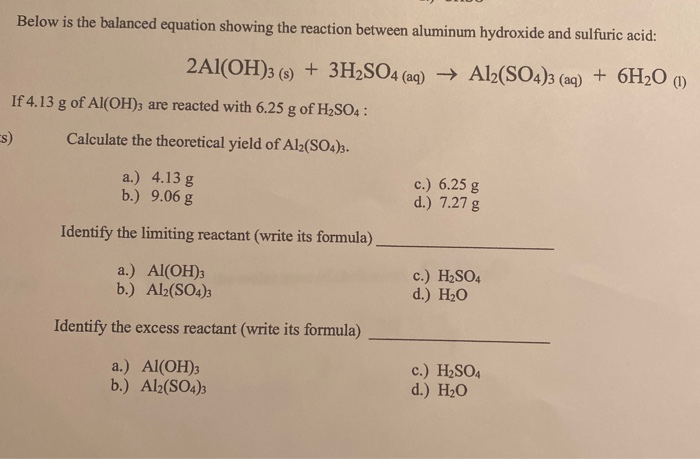
Chem1012 CH04 PowerPoint | PDF | Redox | Acid Aluminum Hydroxide + Sulfuric Acid = Aluminum Sulfate + Water

Source Image: scribd.com
Download Image
Aluminium reacts with sulfuric acid to form aluminium sulfate and hydrogen. What is the volume of hydrogen gas in liters (L) produced – Sarthaks eConnect | Largest Online Education Community Related questions Question Explain this Transcribed Image Text: Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 → Al2 (SO4)3 + 3 H2 Determine how many atoms of each element are present on each side of the arrow. Al: H: S: O: Expert Solution Trending now This is a popular solution! Step by step
Source Image: sarthaks.com
Download Image
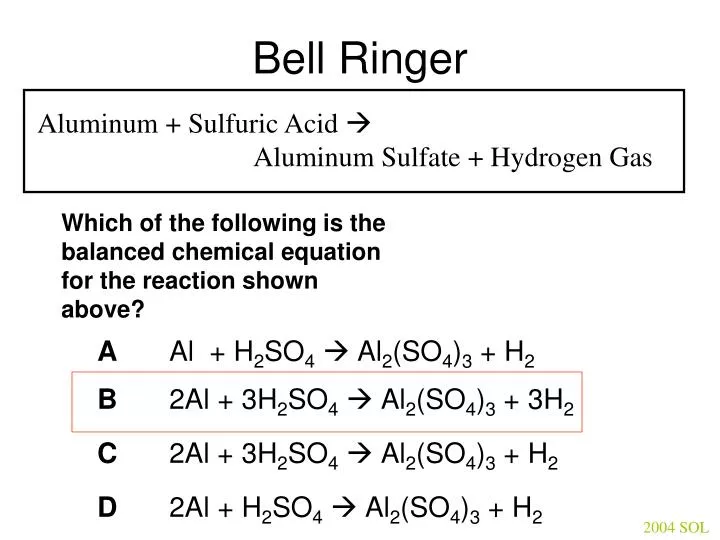
PPT – Bell Ringer PowerPoint Presentation, free download – ID:4423669 Science Chemistry Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 Al (SO4)3 + 3 H2 Determine how many atoms of each element are present on each side of the arrow.
Source Image: slideserve.com
Download Image
Solved Below is the balanced equation showing the reaction | Chegg.com
PPT – Bell Ringer PowerPoint Presentation, free download – ID:4423669 Chemistry Chemistry questions and answers Consider the balanced equation of aluminum reacting with sulfuric acid. 2 Al + 3 H2SO4 + Al2 (SO4)3 + 3H2 Determine how many atoms of each element are present on each side of the arrow. Al: 4 H: 12 (X) S: 6 X 0: 24 This problem has been solved!
How to Write the Net Ionic Equation for Al + H2SO4 = Al2(SO4)3 + H2 – YouTube Aluminium reacts with sulfuric acid to form aluminium sulfate and hydrogen. What is the volume of hydrogen gas in liters (L) produced – Sarthaks eConnect | Largest Online Education Community Aluminum Hydroxide + Sulfuric Acid = Aluminum Sulfate + Water