Figure 7.9.1: Reaction between zinc and sulfur. Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced. An oxidation-reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another.
Redox Reactions Quiz – Trivia & Questions
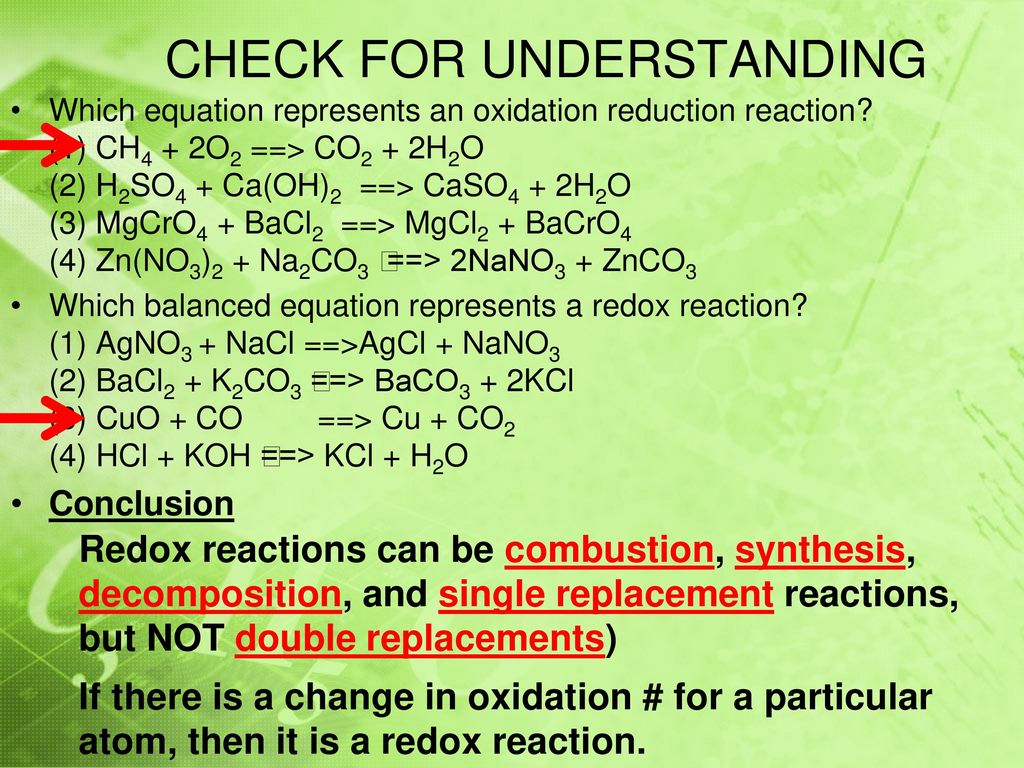
Study with Quizlet and memorize flashcards containing terms like Which balanced equation represents a redox reaction? (1) Mg + Cl2 ==> MgCl2 (2) CaO + H2O ==> Ca(OH)2 (3) HNO3 + NaOH ==> NaNO3 + H2O (4) NaCl + AgNO3 ==>AgCl + NaNO3, In a redox reaction, the number of electrons lost is equal to the number of (1) protons lost (3) neutrons gained (2) neutrons lost (4) electrons gained, In a redox

Source Image: slideplayer.com
Download Image
A powerful technique for balancing oxidation-reduction equations involves dividing these reactions into separate oxidation and reduction half-reactions. We then balance the half-reactions, one at a time, and combine them so that electrons are neither created nor destroyed in the reaction.

Source Image: pinterest.com
Download Image
What is the equation of glycolysis? – Quora Oxidationreduction reactions are vital for biochemical reactions and industrial processes as well. Redox reactions are used to reduce ores to obtain metals, to produce electrochemical cells, to convert ammonia into nitric acid for fertilizers, and to coat compact discs. This is an introduction to oxidation-reduction reactions, also known as

Source Image: vectorstock.com
Download Image
Which Equation Represents An Oxidation Reduction Reaction
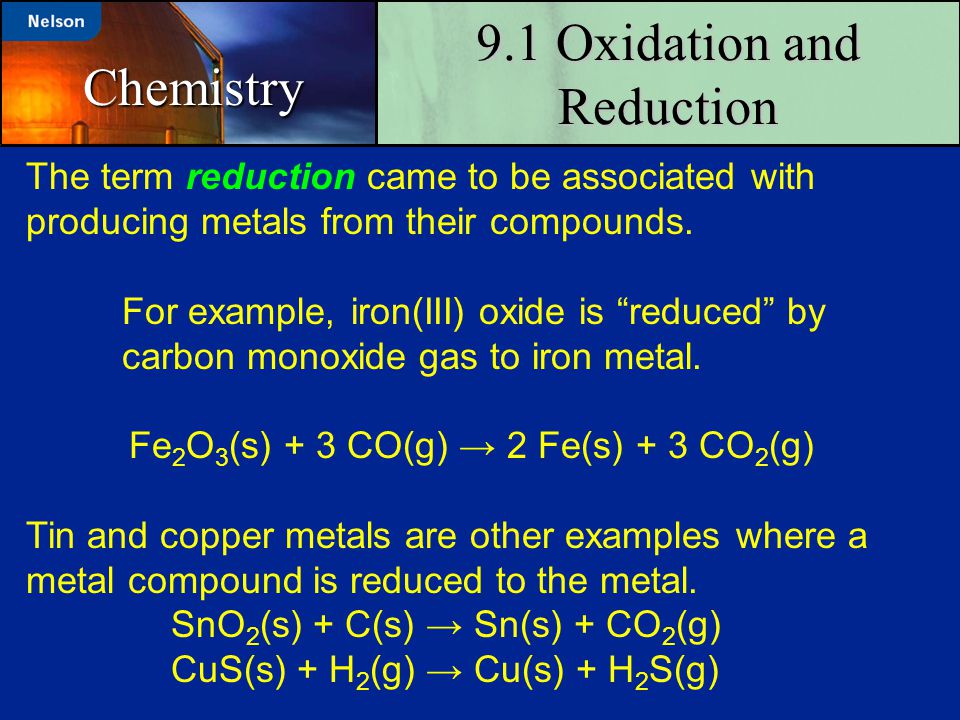
Oxidationreduction reactions are vital for biochemical reactions and industrial processes as well. Redox reactions are used to reduce ores to obtain metals, to produce electrochemical cells, to convert ammonia into nitric acid for fertilizers, and to coat compact discs. This is an introduction to oxidation-reduction reactions, also known as Reduction–oxidation reactions are often called redox equations. A reduction reaction is only one half of a redox reaction. The other half is the oxidation reaction. Consider the single replacement (displacement) reaction between zinc metal and hydrochloric acid: Zn(s) + 2H Cl(aq) → ZnCl2(aq) +H 2(g) The ionic equation for this reaction:.
Oxidation and Reduction Vector Images (72)
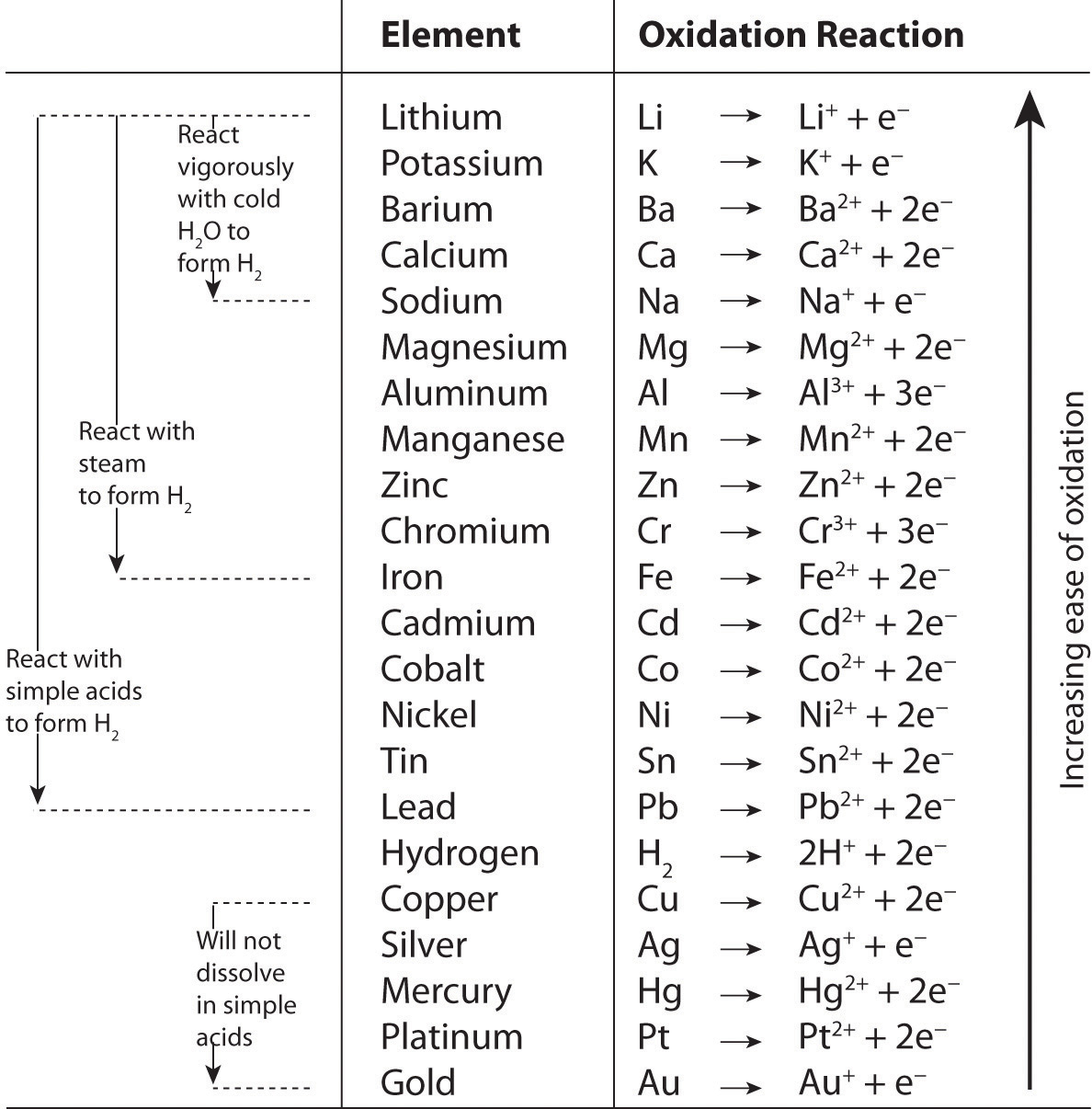
Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. Oxidation is the loss of electrons. Reduction is the gain of electrons. Oxidation and reduction always occur together, even though they can be written as separate chemical equations. Unit 9: Oxidation, Reduction, and Electrochemistry – ppt download
Source Image: slideplayer.com
Download Image
Note 04 (Oxidation Reduction) PDF | PDF | Redox | Ion Chemical reactions in which electrons are transferred are called oxidation-reduction, or redox, reactions. Oxidation is the loss of electrons. Reduction is the gain of electrons. Oxidation and reduction always occur together, even though they can be written as separate chemical equations.

Source Image: scribd.com
Download Image
Redox Reactions Quiz – Trivia & Questions Figure 7.9.1: Reaction between zinc and sulfur. Since the zinc is losing electrons in the reaction, it is being oxidized. The sulfur is gaining electrons and is thus being reduced. An oxidation-reduction reaction is a reaction that involves the full or partial transfer of electrons from one reactant to another.
(397).jpg)
Source Image: proprofs.com
Download Image
What is the equation of glycolysis? – Quora A powerful technique for balancing oxidation-reduction equations involves dividing these reactions into separate oxidation and reduction half-reactions. We then balance the half-reactions, one at a time, and combine them so that electrons are neither created nor destroyed in the reaction.
Source Image: quora.com
Download Image
Chapter 9 Redox Reactions – ppt download Chapter 4. Chemical Reactions and Equations Oxidation-Reduction Reactions Learning Objectives Define oxidation and reduction. Assign oxidation numbers to atoms in simple compounds. Recognize a reaction as an oxidation-reduction reaction. Consider this chemical reaction: 2Na (s) + Cl 2 (g) → 2NaCl
Source Image: slideplayer.com
Download Image
Redox Reaction | PDF Oxidationreduction reactions are vital for biochemical reactions and industrial processes as well. Redox reactions are used to reduce ores to obtain metals, to produce electrochemical cells, to convert ammonia into nitric acid for fertilizers, and to coat compact discs. This is an introduction to oxidation-reduction reactions, also known as

Source Image: scribd.com
Download Image
Oxidation–Reduction Reactions in Solution Reduction–oxidation reactions are often called redox equations. A reduction reaction is only one half of a redox reaction. The other half is the oxidation reaction. Consider the single replacement (displacement) reaction between zinc metal and hydrochloric acid: Zn(s) + 2H Cl(aq) → ZnCl2(aq) +H 2(g) The ionic equation for this reaction:.
Source Image: saylordotorg.github.io
Download Image
Note 04 (Oxidation Reduction) PDF | PDF | Redox | Ion
Oxidation–Reduction Reactions in Solution Study with Quizlet and memorize flashcards containing terms like Which balanced equation represents a redox reaction? (1) Mg + Cl2 ==> MgCl2 (2) CaO + H2O ==> Ca(OH)2 (3) HNO3 + NaOH ==> NaNO3 + H2O (4) NaCl + AgNO3 ==>AgCl + NaNO3, In a redox reaction, the number of electrons lost is equal to the number of (1) protons lost (3) neutrons gained (2) neutrons lost (4) electrons gained, In a redox
What is the equation of glycolysis? – Quora Redox Reaction | PDF Chapter 4. Chemical Reactions and Equations Oxidation-Reduction Reactions Learning Objectives Define oxidation and reduction. Assign oxidation numbers to atoms in simple compounds. Recognize a reaction as an oxidation-reduction reaction. Consider this chemical reaction: 2Na (s) + Cl 2 (g) → 2NaCl